¶ Testing Requirements
Testing Requirements are set by the Oregon Health Authority's (OHA's) administrative rules and can be found on their page. It is important to know and understand these requirements to avoid transferring or selling untested product to retailers and consumers. From time to time, OHA publishes Informational Bulletins relating to specific testing topics.
The following guidance documents are to assist in the understanding of Cannabis testing rules beginning March 31st, 2022. The guidance documents should not be relied on solely. The rules may be reviewed in full on the Rules and Statutes page.
• Timeline of changes (pdf)
• Information Bulletin 2022-01 - Summary of new cannabis testing rules effective March 31, 2022 (pdf)
• Cannabis failed testing guide (pdf)
• Cannabis sampling guide (pdf)
• Cannabis Testing FAQs (pdf)
• Cannabis Testing Quick Guide (pdf)
The latest Sampling and Testing Guide also covers specific guidelines regarding pulling and submitting samples for testing.
Timeline of Older Rule Changes
• All test results recorded on or after February 1, 2020 must also have the Certificate of Analysis (COA) uploaded with the test results except those outlined in Compliance Bulletin CE2021-03: Testing.
• As of January 1st, 2019 Test results no longer expire
¶ Subcontracting
Labs may subcontract testing in accordance with Oregon Administrative Rules. When subcontracting tests in Metrc the subcontracting lab enters the results for the lab test that they were subcontracted for. However, it is the role of the primary lab to record the final results and upload the COA into Metrc.
¶ Final Product Testing
Products are required to be tested for the full range of compliance tests, for sale to consumer, in the final state that it is going to be sold to a consumer. For example, you have 2 separate, fully tested cannabis oils and blend them together. You cannot consider the blended oil as a tested product, it will need to be tested once the two oils are blended together. This is an important clarification so that licensees can save themselves duplicative testing during processing. As a further example, lets say you have your blended oils in bulk, and now you are wanting to put it into vape pens, cartridges, etc. Do each of those pens, cartridges, etc. need to be tested? The short answer is no, the testing can occur at the bulk level and then divided up into the pens and cartridges as long as no added substances or further processing occurs between the bulk oil, and the placing it in the delivery mechanism.
This topic was discussed on Episode 3 of the In The Weeds Podcast, produced by OLCC.
¶ Control Studies
This section has been updated to align with OHA Rule effective March 31, 2022
Due to the changes being made to sampling, control studies are being eliminated from rule. Rules around batching and sampling may be found under OAR 333-007-0350 and 333-007-0360. The Sampling and Testing Guide and the Metrc guide may also help explain the sampling changes in more detail. Guides may be found at The Oregon Health Authority's Website.
¶ Verifying Results Before and After Transfer
It is very important that both the sender and receiver of packages in Metrc take the time to confirm the test status and test results input into the CTS on a package before the package is sent and received. Failure to do so could result in a potential violation notification email being generated and sent to both the sender and receiver of the product. Please note that a TestPassed Status just indicates that all tests that have been conducted have passed, it does not indicate that all required compliance tests for that marijuana item have been completed.
To confirm the test results follow these easy steps:
First find the manifest in your "Licensed Transfers" Section. Using the black triangle on the far left, you can drill down into the manifest to view the stops on that manifest. Using the black triangle next to the facility on manifest you can then drill down to view the packages going to that facility:
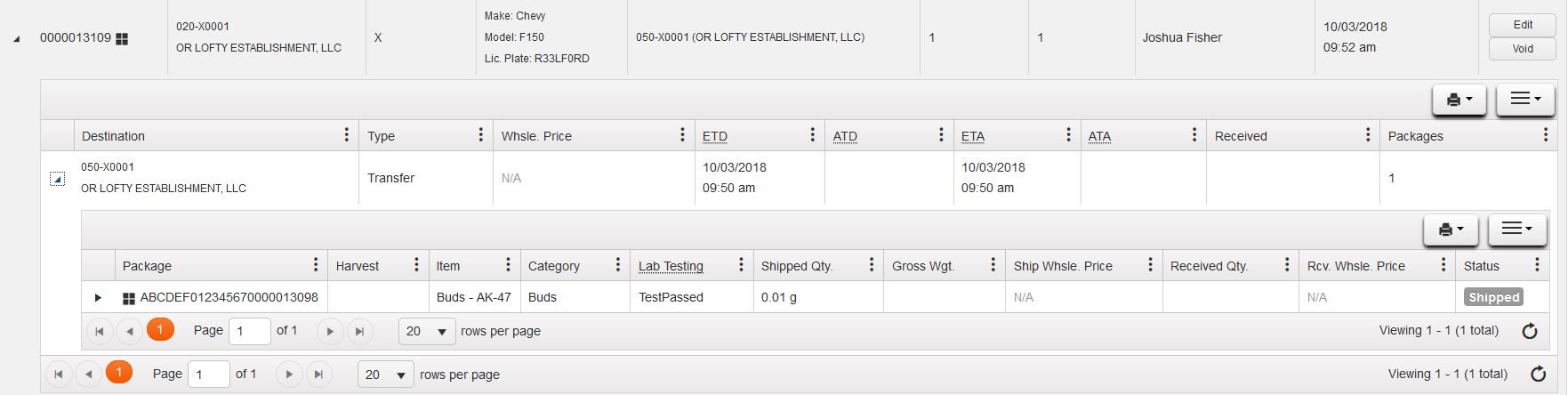
Using the black triangle next to the facility you can then view the lab results tab on that package and view the individual tests that have been completed on that product. In this case, the package is missing several tests and requires more testing before being sent and received by the retailer:
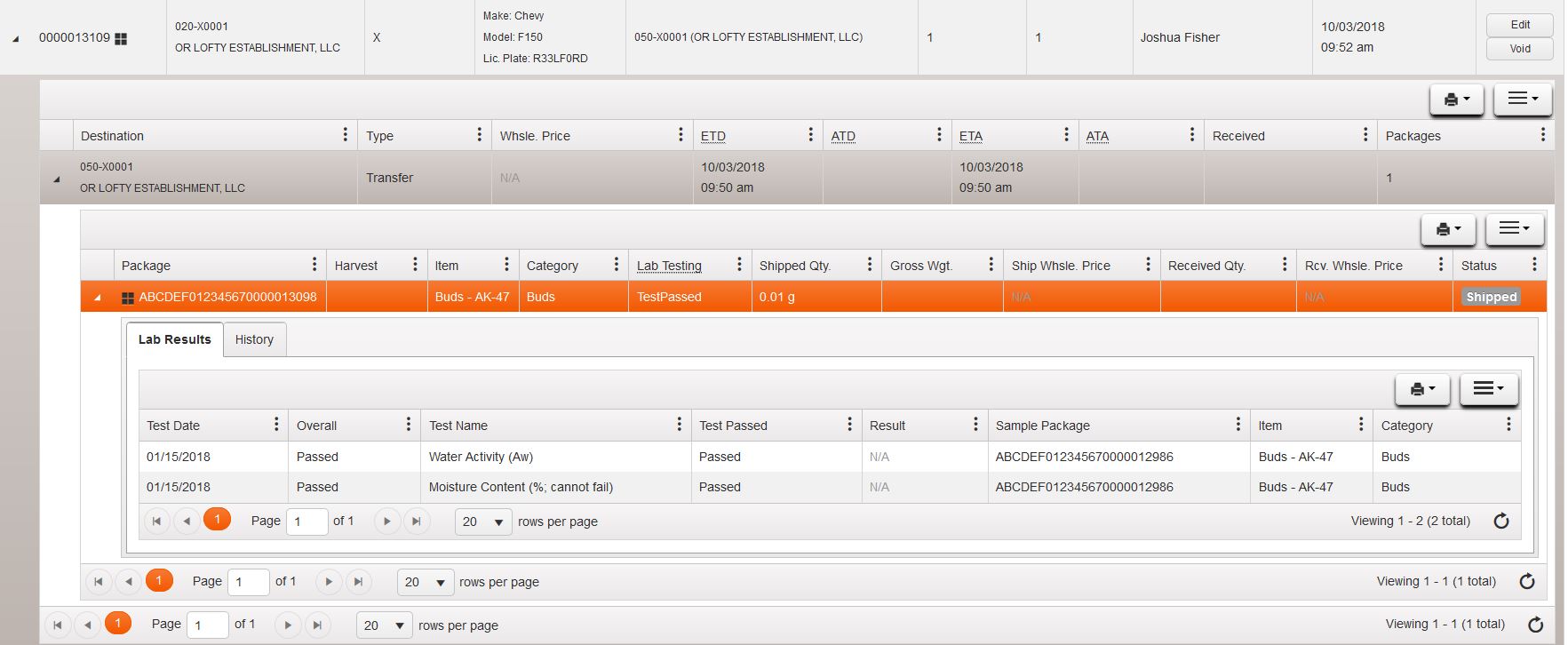
¶ Failed Test Guidance
¶ Reanalysis
Growers and processors who request testing on a marijuana item and receive a failed result may request a reanalysis of the sample from the same laboratory within seven days of being notified of the failure. If a request for reanalysis is made, the grower or processor must notify the OMMP of the request on the Notification of Reanalysis or Retesting of a Failed Sample form (pdf). A copy of the reanalysis results must also be submitted to OMMP once received from the testing laboratory.
- If the reanalysis results in another fail, all associated batches must be held for destruction.
- If the reanalysis results in a pass, then another laboratory must resample and retest the item to determine if the product is a pass or a fail.
¶ Retesting
¶ Recreational
To notify the OLCC follow these instructions:
- Email [marijuana@olcc.oregon.gov](mailto:Marijuana@olcc.oregon.gov?subject=Request for Reanalysis)
- Use the subject line: “Request for Reanalysis”
- Include the following information:
- Your license number and facility name (as they appear in Metrc)
- The Metrc package tag ID of the sample package being reanalyzed
- The lab doing the reanalysis
- Date of request for reanalysis
- Test(s) requested for reanalysis
¶ Medical
The grower or processor must notify OMMP that they are pursuing a retest. The same Notification of Reanalysis or Retesting of a Failed Sample Form (pdf) may be used. A copy of the retesting results must also be submitted to OMMP once received from the testing laboratory.
- A Fail on a retest means the item must be held for destruction.
- A pass on a retest means the item may be transferred.
If a batch fails testing, it may not be destroyed without first obtaining permission from OMMP.
The form and copy of the test results may be sent to: ommp.labs@state.or.us.
¶ Unexpected Results
There have been instances when Licensees have reached out seeking guidance on "unexpected" test results, i.e. for formulated items that have an expected potency level that comes in with a wild variance to "normal" results. Unfortunately, there is no remedy for this in rule. Once compliance testing has been completed, no repeat lab tests can be done on the same batch (with the exception of failed, remediated product). The Best Practice advice for licensees is to remain consistent with the lab utilized for testing to ensure that uniform procedures are followed for every batch. If a licensee feels that there is an egregious error, they could have the product "R&D" tested and try to work with the Oregon Health Authority to build a case for rule changes, but at this time, there is no recourse to amending a test due to undesired results therefore there is no Metrc remedy for this situation.
¶ Selling Hemp Products in Oregon
Hemp Testing and Potency Quick Reference Guide. The Oregon Liquor and Cannabis Commission (OLCC) and the Oregon Department of Agriculture (ODA) receive numerous questions regarding testing and potency limits for hemp items in Oregon. These requirements depend on who manufactures the hemp item and where the item will be sold. OLCC and ODA have put together a short handout summarizing this information for easy reference.
¶ Reporting Lab Issues
When assistance is needed with a sampling issue or you have identified a testing issue that requires a manual override of testing results, obtain a case number from Metrc Support, then submit your request to OLCC using the Lab Issue Report Form or by scanning the QR code below.
Failure to enter valid email addresses for the lab and client will prevent the form from being submitted. Ensure all required fields are completed with valid information.

¶ Common Issues
-
If the lab testing status is "NotSubmitted" check if there are R&D results in the lab results after compliance testing. If so, this issue can be avoided by entering R&D results into Metrc prior to entering compliance results.
-
If the lab testing status is "SubmittedForTesting" or "TestingInProgress" check the package history for a related sample that has not had results posted. The status will update in Metrc once the missing results are posted, please do not submit this form. If that other sample was created on accident and discontinued or destroyed select the option "Multiple samples were taken from the same source/related packages are still in testing" and explain that another sample was created in Metrc but has since been destroyed.
¶ Things to Note
-
OLCC cannot correct category or name issues.
-
Compositing issues are typically complicated to correct and often require several days to resolve.
¶ Wiki Feedback
If you encounter a technical problem or just want to provide general comments regarding your visit to the Oregon Metrc Wiki, we welcome your Feedback.